*
More FIX on the NET @ FIX University Cultural Campus
Using Radiocarbon Dating to Establish the Age of Iron-Based Artifacts
and Jeffrey Wadsworth
 |
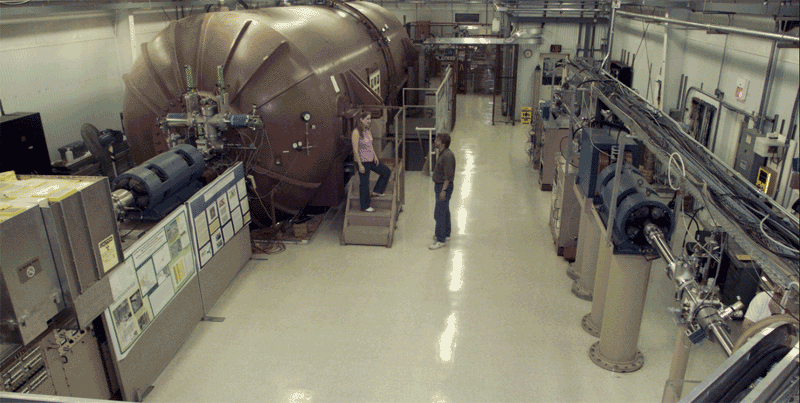
Dawn Ueda and Tom Brown next to the accelerator mass spectrometer at Lawrence Livermore Laboratory. (Photo courtesy of the Center for Accelerator Mass Spectrometry at Lawrence Livermore National Laboratory.) |
| Over the last 40 years, there has been a discernible increase in the number of scholars who have focused their research on early industrial organizations, a field of study that has come to be known as Archaeotechnology. Archaeologists have conducted fieldwork geared to the study of ancient technologies in a cultural context and have drawn on the laboratory analyses developed by materials scientists as one portion of their interpretive program. Papers for this department are solicited and/or reviewed by Michael Notis, a professor and director of the Archaeometallurgy Laboratory (www.Lehigh.edu/~inarcmet) at Lehigh University. |
 |
Figure a. Corroded iron from the Java Sea Wreck. |
 |
Figure b. Chinese Warring States arrowhead dating to about 400–200 B.C. Dawn Ueda and Tom Brown next to the accelerator mass spectrometer at Lawrence Livermore Laboratory. (Photo courtesy of the Center for Accelerator Mass Spectrometry at Lawrence Livermore National Laboratory.) |
 |
| Figure c. A wrought-iron Roman cleaver. |
 |
| Figure d. Large spear from Burkino Faso, Africa. |
 |
| Figure e. Paperweight made by reworking iron from the Himeji Castle in Japan. |
status of the radiocarbon dating of iron-based materials. Recent advances
include simplification in sample preparation and reduction in sample size for
accelerator mass spectrometry measurements, and the potential use of rust as a
viable source of material for radiocarbon dating. Additionally, a summary is
presented of all 63 previously published results for iron-based materials and 29
new results that have not been published previously. These materials range from
low-carbon wrought irons to medium to very high-carbon steels and cast irons.
Artifact dates range from several hundred years ago to several thousand years
ago. Brief descriptions are given of some of these examined samples to
illustrate issues and complexities that can arise in determining the age of
iron-based carbon materials using radiocarbon dating.
INTRODUCTION
As a society, we are deeply interested in determining the age of things, from
the history of our own universe to the details of how modern technological
developments have taken place. A recent summary has been published1 of techniques for dating that
range from astronomical methods to cover time scales from the age of the
universe (e.g., 13 billion years ago) to forensic entomology to determine when
people died (e.g., times on the order of hours).
One well-known method
for dating is based on the use of isotopic techniques. Included are reactions
such as the uranium-to-lead transformation utilized for dates that range from 1
billion years to 4.5 billion years. Perhaps the best-known isotopic technique,
however, is that of radiocarbon [e.g., carbon 14 (14C)] dating, which is used to cover time periods from
several hundred years ago to about 50,000 years ago.
The present paper
deals with an issue of great interest to materials scientists and
archeologists—the dating of iron-based materials that contain carbon. The phrase
“iron-based materials” is used to cover the three common groups of irons and
steels: wrought irons, which are typically low carbon (e.g., less than about
0.05% carbon), steels (up to 2.1% carbon), and cast irons (over 2.1% carbon). In
addition, however, the corrosion products or rust from these materials is
included since they can also be used for dating in some cases. For the case of
iron-based materials, the time span of interest is from the start of the Iron
Age in the regions of interest (about 2000 B.C. or earlier) to several hundred
years ago. The most appropriate method for this time span and group of materials
is 14C dating. It is key to point out that the
usefulness of the method of dating carbon in iron-based materials relies on the
source of the carbon found in the materials (see sidebar).
RADIOCARBON DATING IRON-BASED MATERIALS
Techniques and Their Limitations
The concept of using
radiocarbon dating to determine the age of carbon-containing materials was first
proposed in the 1950s. For the case of iron-based materials, van der Merwe and
Stuiver2 first demonstrated
that it was feasible to extract the carbon from different iron-based materials
and use it to establish their age using radiocarbon dating. A total of 15
samples of iron-based materials were dated by beta counting at Yale University2,3 using a
dependable method to extract carbon from iron utilizing flow-through combustion
in oxygen with cryogenic trapping of CO2. These
studies showed that in a wide range of cases, the carbon in iron-based materials
could be extracted and reliably radiocarbon dated.
The Yale beta counter, however,
required significant amounts of carbon compared to the amounts that were usually
available from artifacts without consuming or damaging them. The amount of
carbon required was 1g, equivalent to 50 g of a 2.0% carbon steel or cast iron,
or 1,000 g of a 0.1% carbon iron, assuming 100% yields in the experimental
process of extracting the carbon.
In the late 1980s, radiocarbon dating
by accelerator mass spectrometry (AMS) became common. This new methodology
required only 1 mg instead of 1 g of carbon. Cresswell4 miniaturized van der Merwe’s extraction
technique and dated 12 different iron artifacts.4–8 Sample sizes in these studies ranged from 3.4
g for an iron bloom (0.4% carbon) to 274 mg for a high-carbon (1.79% carbon)
wootz steel.
In 2001, the present authors published9 a new carbon-extraction method for iron based on
a sealed-tube combustion with CuO in quartz. This greatly simplified the
previous technique and required only materials readily available in the standard
AMS graphite-preparation laboratory: quartz tubes, CuO, vacuum lines, and a
standard electric furnace capable of reaching 1,000°C. Unlike the previous
techniques, no exotic gas-trapping equipment is required.
Thus, over the
years, the sample-size requirement has been greatly reduced and the
carbon-extraction procedure has been simplified. However, as has been mentioned,
for a radiocarbon date on iron to be meaningful, the carbon extracted from the
iron-based material must be from biomass contemporaneous with original
manufacture. In addition to fossil fuels such as coal and coke, other carbon
sources such as geological carbonates (e.g., limestone and siderite), shell, or
old wood (which are all depleted in 14C) will
cause artifacts to appear to be older than they are. Complications arising from
the recycling of artifacts must also be considered. These limitations of the
dating technique have been well summarized by van der Merwe3 and Cresswell.5
Radiocarbon Dating of
Rust
A second interesting area concerns the use of rust for dating.
If rust can be dated reliably, it opens up a large number of possibilities for
dating iron artifacts. Investigators will not need to cut into valuable
artifacts for clean metal, but will be able to use surface corrosion products.
This potentially opens the way for dating precious samples such as the iron
plate found in the Great Pyramid at Gizeh,10,11 now at the British
Museum. It may also be possible to date completely rusted artifacts, commonly
found in waterlogged early Iron-Age sites in Europe and in underwater
shipwrecks. Previous investigators had been careful to remove rust from iron
prior to dating for fear that it adds contamination. A key issue though, is
whether any of the original carbon remains within the matrix of rust and other
corrosion products. If not, rust and similar materials are clearly of no
interest for radiocarbon dating and should probably be removed since, at best,
they can do no good. However, if original carbon is present, the corrosion
products themselves may be appropriate targets for dating, subject to solving
the potential contamination problems.
Most of the carbon in iron-based
materials is in the form of the orthorhombic, crystalline iron carbide (Fe3C) known as cementite. Morphologically, cementite
appears either as spheroidized particles or as pearlite. For compositions
exceeding the eutectoid level of about 0.8% carbon, the excess carbide often
exists as massive plates of proeutectoid cementite. The thickness and sizes of
all of these carbides can vary enormously, depending upon composition and
heat-treatment history. For steels that have been quenched to form martensite
(body-centered tetragonal structure), the carbon is essentially in solid
solution in the iron up to the eutectoid composition, beyond which it too will
usually be in the form of carbides.
Despite the complex range of possible
amounts and morphologies of the cementite, the thermodynamic stability of iron
carbide is significantly greater than that of iron. So, as iron rusts, the
carbide phase will be more stable than the matrix and will remain behind. The
question then becomes one of kinetics: How long will it take for the carbide to
oxidize compared to the iron matrix? As long as the carbon remains in the rust,
in whatever form, it will potentially be available for radiocarbon dating.
Although little appears to have been published on this subject,
Knox12 reported the
detection of iron carbide in the remaining oxide from a corroded 2,800-year-old
Iranian steel dagger.
Optical metallography explicitly showed the
presence of “unaltered” iron carbides in the rust from this object. Also present
in the microstructure were pseudomorphs of carbides, which had been reduced by
corrosion to carbonaceous regions that were “probably an intimate mixture of
oxide and amorphous carbon.” More recently, Notis13 has succeeded in making carbon maps in rusted
old steel samples using electronprobe microanalysis techniques at Lehigh University and has
observed good carbon images in the microstructures.
The present authors
and van der Merwe14 have
recently completed a study in this area. This work provides some evidence for
the reliability of dating corrosion products from artifacts that have rusted in
the air, in the ground, and under water, although it does not prove that all
such samples can be successfully dated. Nonetheless, iron samples that had
completely rusted produced plausible radiocarbon dates, but issues of
contamination and post-depositional carbon exchange must be thoroughly tested in
a variety of field settings before rust dating can be considered a validated
technique. The present authors’ results indicate, however, that in at least some
circumstances, the original carbon from iron-based materials is retained in rust
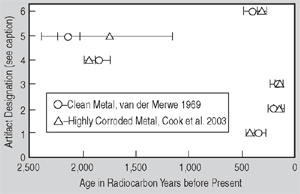
and can be cleanly extracted and dated (see Figure 1a and Figure a).
What the study does show, then, is that there is no a priori reason why the
method should not work on rust. The work suggests that accurate radiocarbon
dates may be obtainable with minimal material and with minimal risk to
artifacts.
| a | b | |
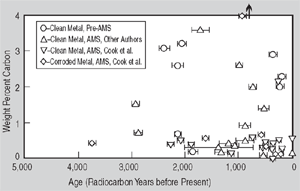
Figure 1. (a) Radiocarbon ages for clean and highly corroded metal: a comparison. Artifacts are designated as follows: (1) Saugus Ironworks, (2) Hopewell Furnace, (3) Redding Furnace, (4) Scottish Iron, (5) Szechwan China, and (6) Hunan China. | Figure 1. (b) A summary of iron artifacts verified by radiocarbon dating: Weight percent carbon vs. age in radiocarbon years before present. | |
OVERVIEW OF PREVIOUSLY PUBLISHED RESULTS
To the best of the authors’ knowledge, Table I lists
all of the radiocarbon data ever published for iron and steel. This table
provides sample identification, radiocarbon years Before Present [B.P.] (Note:
This nomenclature is used in radiocarbon dating to avoid the variation
introduced by calibration charts that convert radiocarbon years B.P. to calendar
years by accounting for variations in the 14C
levels through history20–22) sample size, sample condition, date of
presumed manufacture, calibrated date, method, and reference. Since 1968, a wide
range of iron-based materials have been investigated (Figures a, b, c, d, and e). The determined ages range from relatively
recent materials (350 B.P.) to materials from an era approaching the start of
the Iron Age (4000–5000 B.P.). Materials range from very low-carbon wrought
irons to cast irons (> 2.1% carbon). Sample sizes range from less than 0.05 g
to more than 500 g. Sample conditions range from clean metal to rusty metal to
very corroded metal. In only 11% (7/63) of the cases, complications arose such
that the authors could not explain their data simply. The nuances of radiocarbon
dating of iron-based materials will be explained by way of example in this
paper.
Artifact Identification | 14C B.P. | %C | Sample Size (g) | Presumed Manufacture | |||
| Radiocarbon Dates that Matched the Date of Presumed Manufacture | |||||||
| Saugus Ironworks, MA | |||||||
| Hopewell Furnace, PA | |||||||
| Redding Furnace, PA | |||||||
| Roman fort, Scotland | |||||||
| Han Dynasty, Sian, China | |||||||
| Han Dynasty, Szechwan, China | |||||||
| Warring States Period, Honan, China | |||||||
| La Tène I, Yugoslavia | |||||||
| Sri Lankan wootz steel | |||||||
| ROM Luristan steel dagger | |||||||
| MIT Luristan steel dagger | |||||||
| Japanese sword | |||||||
| Planing adze, China | |||||||
| Ungwana, crucible and bloomery steel (Africa) | |||||||
| Ungwana, crucible steel (Africa) | |||||||
| Ungwana, bloomery steel (Africa) | |||||||
| Ungwana, crucible steel (Africa) | |||||||
| Galu, white cast iron (Africa) | |||||||
| Hook from Horyuji Temple, Japan | |||||||
| Himeji castle nail, small | |||||||
| Damascus knife | |||||||
| Cauldron, Java Sea wreck | |||||||
| Nail, earthquake fault in Turkey | |||||||
| Italian armor (N-7) | |||||||
| Italian armor plate (N-9) | |||||||
| Tie pin, Ipswich, MA (N-12) | |||||||
| Denbigh, VA (N-20) | |||||||
Radiocarbon Dates that Did Not Match the Date of Presumed Manufacture, but Were Easily Explainable by the Authors at the Time of Publication | |||||||
| Modern, coke-smelted cast iron | |||||||
| Fort Kiowa (?), SD | |||||||
| Fort Berthold, ND | |||||||
| Hunan Province, China | |||||||
| Gate from Myohouji Temple, Tokyo | |||||||
| Anchor dedicated to Isonomae shrine | |||||||
| Pail in Inari shrine | |||||||
| Modern steel, 1.3%C | |||||||
| Modern steel, 1.9%C | |||||||
| Italian armor (N-5) | |||||||
| Italian sword (N-8) | |||||||
| German armor (N-6) | |||||||
| German armor (N-11) | |||||||
| Axle Thimble, Fort Lower Brule, SD (N-15) | |||||||
| Fort Atkinson, WI (N-18) | |||||||
| Williamsburg, VA (N-21) | |||||||
| Eylon’s own sample A | |||||||
| Eylon’s own sample B | |||||||
Radiocarbon Dates that Did Not Match the Date of Presumed Manufacture, but Were Not Easily Explainable by the Authors at the Time of Publication | |||||||
| Frobisher bloom #1 | |||||||
| Frobisher bloom #2 | |||||||
| Frobisher bloom #3, near surface | |||||||
| Frobisher bloom #3, 2 cm in | |||||||
| Frobisher bloom #3, 5 cm in | |||||||
| Galu, bloomery steel (Africa) | |||||||
| Galu, crucible steel (Africa) | |||||||
Radiocarbon Dates for Iron-Based Materials That Were Dated More Than Once | |||||||
| Saugus Ironworks, MA | |||||||
| Redding Furnace, PA | |||||||
| Modern, coke-smelted cast iron | |||||||
| Cast iron, Fort Kiowa, SD | |||||||
| Cast iron, Fort Berthold, ND | |||||||
| Cast iron, Sian, China | |||||||
| Cast iron, Saugus MA | |||||||
| Cast iron, Hopewell, PA | |||||||
| Cast iron, Redding Furnace, PA | |||||||
| Bloomery iron, Scotland | |||||||
| Cast iron, Hunan, China | |||||||
| Cast iron, Szechwan China | |||||||
| a—clean metal sample condition; b—extremely corroded sample condition; c— rusty metal sample condition; d—pre-AMS method; e—AMS method; f—coal; g—replica; h—reworking with coal; i—after A.D. 180 | |||||||
OVERVIEW OF NEW RESULTS
Table II lists all of the unpublished radiocarbon data
for iron known by the authors. Most of the data is the authors’, but nine of the
data points come from their predecessor, Richard Cresswell.23 Together, 29 new data points have been
generated for iron-based materials that fall into two distinct categories:
radiocarbon dates that matched the dates of presumed manufacture, and
radiocarbon dates that did not match the dates of presumed manufacture. In only
one case (the nose ring from Burkina Faso, Africa), the authors were not able to
provide a simple explanation for the radiocarbon date obtained. All pertinent
information is provided in Table II. Figure 1b summarizes all of the iron artifacts which have ever
been ratified by radiocarbon dating.
Brief sample descriptions and
commentary are provided in the following.
Gibson axe: What is believed to
be a pick-axe point was found during the 12th season in Nippur, Iraq on the
floor of a temple in Area WA (sample ID 12 N 380). The floor dates to the 19th
century B.C., but it is possible that the axe was intrusive from a 13th century
B.C. level above. The axe was completely corroded. Direct radiocarbon dating on
the rust showed the date of the axe to be in accordance with the date of
presumed manufacture: 1900 B.C. To date, this is the oldest piece of iron-based
material to be radiocarbon dated. It suggests that corrosion products contain
original carbon that can be extracted and reliably radiocarbon
dated.
Wrought-iron cleaver, Roman: This wrought-iron cleaver blade with
handle (Figure c) was purchased from Edgar L. Owen at a special
auction of Roman-period iron tools (www.edgarlowen.com). The piece was reported to be from the
Roman or late Roman period. The radiocarbon date obtained for the cleaver was in
accordance with the date of presumed manufacture.
Wrought-iron nails,
Roman: A large number of wrought-iron nails were recovered in the 1960s from the
legionary Roman fortress at Inchtuthil (which stood only from A.D. 83–87). The
nails are reported to have been made in the nearby forest of Dean at Beauport
Park, East Sussex, Britain in one of the largest iron refineries in the history
of the Roman Empire. The nails were purchased through a catalog by Harlan J. Berk, Ltd. (www.harlanjberk.com). The
radiocarbon date obtained from the Inchtuthil nail was in accordance with the
date of presumed manufacture.
Spear blade, Israel: What is believed to be
a spear blade reportedly from Israel, is dated at about 1000 B.C. It was
purchased from Alex G. Malloy, and had a radiocarbon date in accordance with the
date of presumed manufacture.
Roman-period arrowhead: A Roman-period
arrowhead from the late Roman or Crusader period was also purchased from Alex G.
Malloy. The radiocarbon date obtained for the arrowhead was in accordance with
the date of presumed manufacture.
Burkina Faso, African artifacts (small
spear, large spear, and nose ring): These iron-based artifacts were recovered
during an archeological investigation of various mound complexes in Burkina
Faso, Africa. Each iron artifact was associated with a specific mound layer that
was radiocarbon dated independently using charcoal remains. The radiocarbon
dates obtained for the two spears (the larger one is shown in Figure d) were in accordance with the date of the layer in
which they were found; however, the date for the nose ring was not. The authors
can offer no simple explanation for the date of the nose ring. Perhaps it was
reworked using coal or was somehow contaminated with a petroleum-based oil (or
other product).
Japanese folded steel: This steel was made in A.D. 1995
for use in the traditional Japanese sword-making industry. It was smelted in
small batches using only modern charcoal. The radiocarbon date obtained for the
steel was in accordance with the date of presumed manufacture. This piece of
steel is the youngest iron-based material to ever be radiocarbon
dated.
Japanese tanto tang: Some years ago, an old knife was given to a
Japanese swordmaker (Yoshino Yoshihara) to be reforged and used to refurbish and
repair other blades from the same period. The tang (back end of the knife) is
inscribed with the date A.D. 1539. The swordmaker dismantled the tanto, keeping
the blade for repair work and giving the tang as a gift. The radiocarbon date
obtained for the tanto tang was in accordance with the date of presumed
manufacture.
Himeji castle artifacts (pinch dog, large nail, small
bracket, medium nail, and reforged nail): There have been fortifications in
Himeji since A.D. 1333. Today’s castle was built in A.D. 1580 by Toyotomi
Hideyoshi and enlarged some years later (A.D. 1600–1609) by Ikeda Terumasa for
the Tokugawa Shogunate. The five-story castle has been home to 48 successive
lords. While undergoing restoration about 35 years ago, some iron-based
materials were removed from the castle in Japan.
The authors were able to
obtain four pieces from the castle, plus a nail and a paperweight (see Figure e) that had been reforged (mildly,
using low heat) from castle materials.
In radiocarbon dating these items,
three pieces were found to be in accordance with the original building of the
castle (pinch dog, large nail, small bracket) and the other (medium nail) to be
in accordance with a later remodel. The reforged nail appeared to be almost
2,000 years old, and, therefore, must have been reheated using either coal or a
mixture of charcoal and coal. Since coal was used, the date of original
manufacture for this nail cannot be determined using radiocarbon
dating.
Nikko Shrine, large bracket: The Shrine in Nikko was constructed
as a memorial to the warlord, Tokugawa Ieyasu, whose shogunate ruled Japan for
250 years. Tokugawa Ieyasu was laid to rest among Nikko’s towering cedars in
1617 A.D., but it was his grandson, Tokugawa Iemitsu, who commenced work in 1634
A.D. on the shrine that can be seen today. The original shrine was completely
rebuilt in 1818 A.D. The authors obtained what appears to be an iron brace,
possibly from one of the large doorways in the shrine. It was assumed to
originate with the 1634–1636 A.D. construction. The radiocarbon date, however,
accords better with the 1818 A.D. reconstruction.
Basque nail, Labrador
coast, Canada: A large nail was recovered from Red Bay on the Labrador coast,
Canada, associated with a Basque whaling station based there in the mid-1560s. A
large number of iron artifacts have been recovered,24 some imported, others made on site. The age of
this nail accords well with the postulated origin and was probably brought to
the site from Europe.
Fishbourne nail, Sussex, United Kingdom: Shortly
after the Roman invasion of Britain in the first century, a large villa was
erected at Fishbourne (just west of Chichester, Sussex, United Kingdom). This
burned down in the late 3rd century and was disused until Saxon times when it
became a cemetery. In medieval times it was razed, put under crop, and so
remained until the 19th century.25 The nail was recovered from a large spoil heap
presumed to be of Roman origin. The date, however, accords better with Saxon
origin.
Modern bloom: A modern bloom was smelted in A.D. 1986 at the University of Toronto using
charcoal derived from young saplings. The measured activity agrees within
precision with the expected atmospheric concentration as measured at northern
hemisphere atmosphere stations.26 The bloom is therefore in accordance with the
date of presumed manufacture.
Roman iron, Colona Antonina, Italy: A
wrought-iron railing bar was recovered by La Sapienza University during the A.D.
1985 restoration of the Colona Antonina (Marcus Aurelius Column) in Rome. It was
replaced with a titanium railing. A segment of the iron railing was given to the University of Dayton to
determine through carbon dating and metallography if it was installed during the
original A.D. 180 construction, or when it was restored and “Christianized” in
A.D. 1589.
D. Eylon18 was the first to radiocarbon date the railing
and concluded that it must have been installed in A.D. 1589 because the
radiocarbon ages obtained (see Table I), while not
concurrent with A.D. 1589, were not old enough to be original. Eylon, seeking
clarification, sent the authors a segment of the column to radiocarbon date. The
authors sectioned the bar into three pieces and radiocarbon dated each piece
separately. Three different radiocarbon dates were obtained. In this case, it is
clear that radiocarbon cannot be used to obtain the date of original
manufacture.
This sample exemplifies another category of iron-based
materials for which radiocarbon dating will be unable to determine the date of
original manufacture. The railing was clearly shown by metallography18 to be inhomogeneous and made
of a composite of many different strips of iron welded together. The individual
strips could have been manufactured at any time using any method.
World
War II steel, Marin, California: Steel believed to be from World War II was
collected by one of the authors (A. Cook) from an abandoned military battery in
Marin, California. While the exact manufacturing process is unknown, it was
thought to have been mass produced with coal near the start of World War II. The
radiocarbon date obtained for the iron-based material was in accordance with at
least partial manufacture by coal.
Indian trade axes, Ontario, Canada:
Cahiague Ball: Axes, thought to have been associated with European-Native
American trading in the 19th century, were recovered from Cahiague sites 26712a,
26712b, 26697, and 26698 and Ball 2046 inner and outer sites near Ontario,
Canada. A complete axe head was recovered from the Ball site, while only pieces
of axes were recovered at the Cahiague site. Radiocarbon dating showed the
pieces of axes to have inconsistent dates that were only explained following
examination of the complete axe head.
Metallurgical examination revealed
the axe to be a composite piece of welded strips of iron. Pieces of the inner
and outer strips were separated and analyzed, giving quite distinct radiocarbon
signatures. These axes were clearly the result of significant re-working of iron
from different origins, many being much younger than hoped, and including strips
of iron from coal-fired furnaces. As with the Colona Antonina railing,
radiocarbon dating cannot determine the manufacture date for these objects.
Artifact Identification | 14C B.P. | %C | Sample Size (g) | Presumed Manufacture | ||
| Radiocarbon Dates that Matched the Date of Presumed Manufacture | ||||||
| Gibson axe | ||||||
| Wrought-iron cleaver, Roman | ||||||
| Wrought-iron nails, Roman | ||||||
| Spear blade, Israel | ||||||
| Roman period arrowhead | ||||||
| Burkina Faso, Africa, small spear | ||||||
| Burkina Faso, Africa, large spear | ||||||
| Japanese folded steel | ||||||
| Japanese tanto tang | ||||||
| Himeji Castle, pinch dog | ||||||
| Himeji Castle, large nail | ||||||
| Himeji Castle, medium nail | ||||||
| Himeji Castle, small bracket | ||||||
| Nikko Shrine, large bracket | ||||||
| Basque nail, Labrador coast, Canada | ||||||
| Fishbourne nail, Sussex, U.K. | ||||||
| Modern bloom | ||||||
Radiocarbon Dates that Did Not Match the Date of Presumed Manufacture and are Discussed by the Present Authors | ||||||
| Himeji Castle, reforged nail | ||||||
| Burkina Faso Africa, nose ring | ||||||
| WWII steel, Fort SF | ||||||
| Roman iron, Colona Antonina, Italy | ||||||
| Roman iron, Colona Ant., Italy C | ||||||
| Roman iron, Colona Ant., Italy D | ||||||
| Cahiague 26712a, axe head, Ontario, Canada | ||||||
| Cahiague 26712b, axe head | ||||||
| Cahiague 26697, axe head | ||||||
| Cahiague 26698, axe head | ||||||
| Ball 2046: inner, axe head | ||||||
| Ball 2046: outer, axe head, Ontario, Canada | ||||||
| a—clean metal sample condition; b—extremely corroded sample condition; c— rusty metal sample condition; d—14C dated by Cook et al.; e—14C dated by Cresswell; f—donated by Gibson, University of Chicago; g—donated by Pense, Lehigh University; h—donated by Holl, University of Michigan; i —donated by Kapp, University of San Francisco; Yoshihara, Japan; j —donor not stated in unpublished manuscript; k—donated by the University of Toronto; l—donated by Cook, High Tech High; m—donated by Eylon, University of Dayton; n—reforged using coal; o—no explanation; p—made with coal; q—inhomogeneous; r—reworked | ||||||
CONCLUSIONS
To date (including this paper), a total of 92 different radiocarbon
measurements have been published on iron-based materials. The determined ages
range from very recent materials (1995 A.D.) to materials approaching the
commonly accepted era for the start of the Iron Age (4000–5000 B.P.). Materials
range from very low-carbon wrought irons (0.01% carbon) to cast irons (> 2.1%
carbon). Sample sizes range from less than 0.05 g to more than 500 g. Sample
conditions range from clean metal to very corroded metal and rust.
In
principle, then, there is not a period in Iron-Age history that cannot be
investigated using radiocarbon dating. As long as assumptions hold (the
iron-based material is manufactured using only contemporaneous charcoal—no old
wood, no reworking, no coal, no limestone flux), the radiocarbon dating of
iron-based materials has been shown to be very reliable.
Not
surprisingly, however, there are iron-based materials that are not suited for
accurate dating by radiocarbon. These include materials manufactured using coal
(e.g., most modern steel made after 1800 A.D.), materials reheated using coal
(e.g., reforged Himeji castle nails and possibly the European armor and
Frobisher bloom), and materials that are composites (Indian trade axes, the iron
railing from the Colona Antonina). Although radiocarbon dating cannot be used to
determine the age of these materials, it may, however, yield valuable insight
into the manufacturing processes that were used to create these
materials.
The first warning that an artifact is unsuitable for dating by
radiocarbon is when multiple samples are run and the dates obtained are widely
variable. Reworked samples, especially those reworked with coal, are often
inhomogeneous with respect to the age of the carbon in the metal due to
variations in absorption. Clearly, under these conditions, the iron-based
material is not suitable for dating by radiocarbon. Fortunately, such cases are
usually quite obvious from preliminary results.
In only a very small
fraction of the cases in which iron-based materials have been dated can the data
not be readily interpreted. In most cases, when the radiocarbon date did not
match the date expected, it was possible to deduce something about the
manufacturing process. The Colona Antonina and the Indian trade axes were most
likely reworked composites of iron scraps with different coal/charcoal origins.
The Frobisher blooms seem to have undergone multiple reworking attempts over
time. The European armor investigated may have been replicas, or perhaps coal
was used in the process of making European armor earlier than was previously
thought. On the other hand, the authors do not understand why the nose ring from
Burkina Faso appears to be so old. There is no simple explanation. Either the
sample is contaminated or perhaps old carbon was somehow used in the
manufacturing process. Either way, radiocarbon is not likely to help in
determining the exact date of manufacture for these objects. As discussed,
nuances and complications still exist in interpreting the radiocarbon dates from
iron-based materials; however, many aspects are becoming better understood as
more samples are examined.
It is important to note that with the large
reduction in required sample size and the recent discovery that rust (at least
in some cases) can be reliably dated, the door has opened for investigations on
the very earliest iron, dating from 1000 B.C. and earlier. It is of no surprise
that this period contains the fewest data, and the present results suggest there
is no a priori reason to believe that items from this time period cannot be
dated reliably with radiocarbon.